
In liquids, the particles are touching but are free to move around each other like balls in a ball pool.
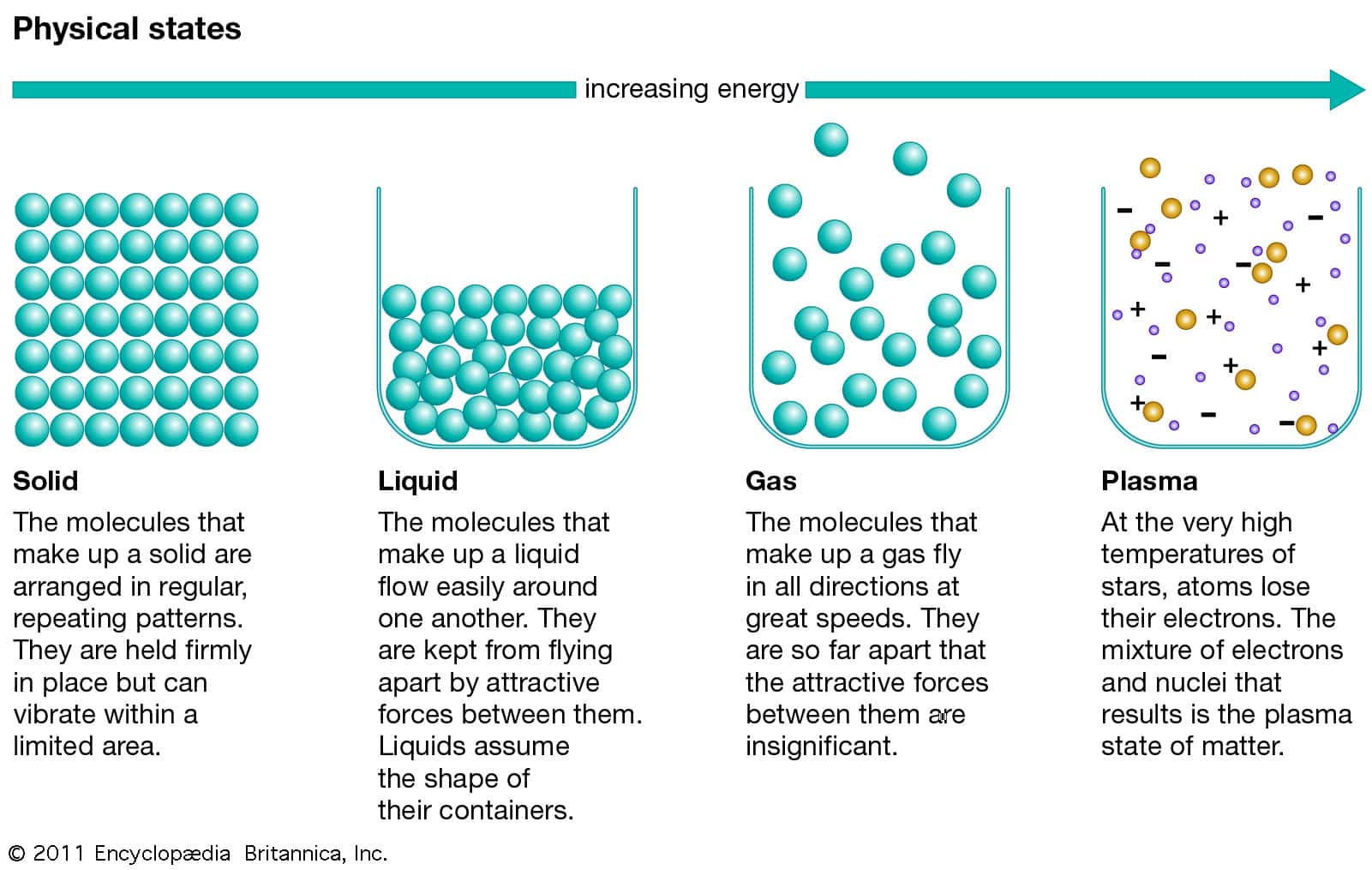
In this model, solids have particles fixed tightly together in rows so they can’t move. We tend to understand this using a simplified model in which we imagine matter is made of spherical particles. Gases, on the other hand, expand to fill whatever container they are placed in. Liquids have a defined volume but will flow into containers and take on their shape. Solids have their own defined shape that won’t change without an external force being applied. These are categories of stuff that have certain observable properties.

It turns out that any material, no matter what it is made of, can exist in one of three forms: solid, liquid or gas. The state of matter is a deceptively simple concept.


 0 kommentar(er)
0 kommentar(er)
